what is the atomic number
Hey friends, today we are going to talk about an interesting topic – The Atom! At the very center of all matter lies the atom. It’s the basic unit of a chemical element, and is defined as the smallest particle of matter that retains the chemical properties of the element. So, let’s take a closer look at what makes up an atom, its various components, and what makes it so important.
The Structure of an Atom
Abstract:
The structure of an atom is made of three fundamental particles – protons, neutrons, and electrons. Protons, which have a positive charge, along with electrons, which have a negative charge, are found in the atom’s nucleus. Neutrons, which have no electric charge, are also found in the nucleus. The number of protons is what gives an atom its identity, and is known as its atomic number. The number of protons and neutrons together make up the atom’s mass number. Different isotopes of the same element have the same number of protons but different numbers of neutrons.
Introduction:
Now, let’s take a closer look at each of these particles and their properties.
Protons:
Protons have a positive charge and are located in the nucleus of the atom. They are identified by their atomic number, which is the number of protons in the nucleus. The atomic number determines the identity of the element. For example, all carbon atoms have six protons, and all nitrogen atoms have seven protons.
Neutrons:
Neutrons have a neutral charge, and are also located in the nucleus. They help hold the nucleus together by overcoming the repulsion between positively charged protons. The number of neutrons can vary among different atoms of the same element, and atoms with the same number of protons but different numbers of neutrons are called isotopes of that element.
Electrons:
Electrons have a negative charge, and are found outside the nucleus in various energy levels, or shells. The number of electrons in an atom is equal to the number of protons, and when an atom loses or gains an electron, it becomes a charged particle called an ion.
The Atomic Number
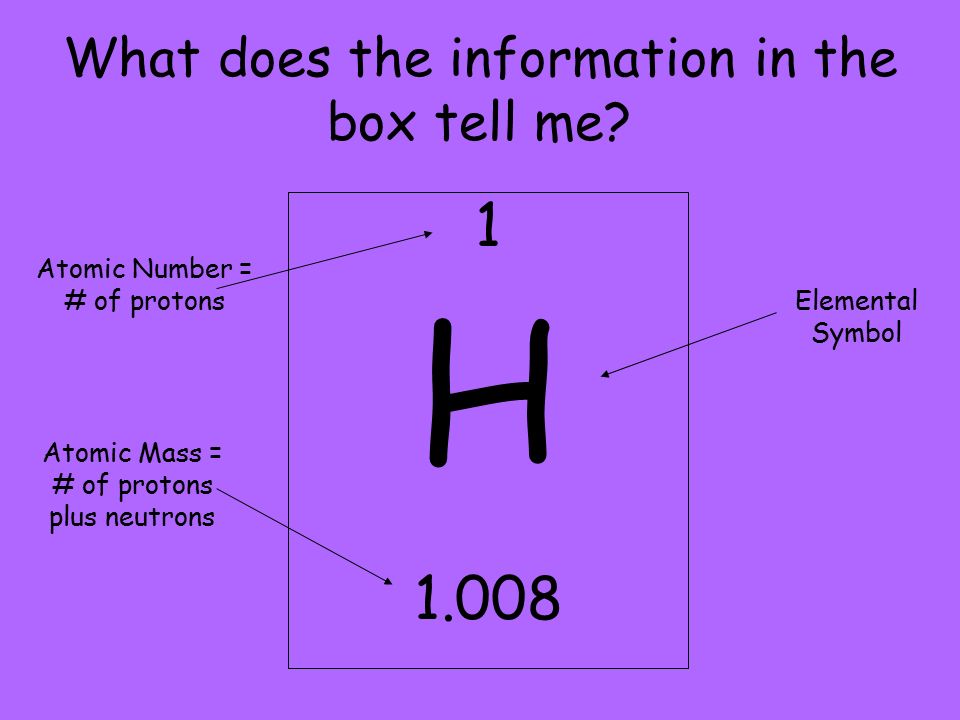
Abstract:
The atomic number is the number of protons in the nucleus of an atom, and is represented by the letter Z. The atomic number determines the identity of an atom, and is unique to each element. For example, all carbon atoms have an atomic number of 6, because they have six protons in their nucleus. The number of neutrons can vary among different atoms of the same element, and atoms with the same number of protons but different numbers of neutrons are called isotopes of that element.
Introduction:
Now, let’s take a closer look at how the atomic number and isotopes affect the properties of atoms.
Atomic Number and Chemical Properties:
The atomic number determines the identity of an atom, and is used to categorize elements in the periodic table. Elements with the same number of protons have similar chemical properties, and often have the same number of valence electrons, which are the electrons in the outermost shell that determine how an atom will react with other atoms to form compounds. For example, all elements in Group 1 of the periodic table have one valence electron, and tend to form compounds that are ionic, or positively charged.
Isotopes and Nuclear Stability:
Isotopes of the same element have the same number of protons, but different numbers of neutrons in the nucleus. Some isotopes are stable, meaning they don’t undergo radioactive decay, while others are unstable, or radioactive. The stability of an isotope depends on the balance between the forces that hold the nucleus together and the forces that try to push it apart. Some isotopes are naturally occurring, while others are artificially produced in nuclear reactors or particle accelerators.
Atomic Mass

Abstract:
The atomic mass of an atom is the mass of its nucleus, which is made up of protons and neutrons. It is represented by the letter A, and is approximately equal to the number of protons and neutrons in the nucleus. However, because electrons have such a small mass, the atomic mass is almost entirely made up of the mass of the nucleus. The mass of an atom is important in many chemical and physical processes, such as determining the amount of reactants needed to produce a certain amount of product, or calculating the kinetic energy of gas particles.
Introduction:
Now, let’s take a closer look at how atomic mass is calculated, and how it relates to the properties of elements.
Calculating Atomic Mass:
The atomic mass of an element is calculated by adding the number of protons and neutrons in the nucleus. For example, carbon-12, which is the most common isotope of carbon, has 6 protons and 6 neutrons, giving it an atomic mass of 12. Some elements have multiple isotopes, which are defined by their atomic mass number. The average atomic mass of these isotopes is calculated by multiplying the mass of each isotope by its percentage in nature, and then adding up the results. This is known as the weighted average atomic mass.
Relationship between Atomic Mass and Properties:
The atomic mass of an element affects many of its physical and chemical properties. For example, elements with higher atomic masses tend to have higher melting and boiling points, because the stronger forces between the particles require more energy to overcome. Elements with lower atomic masses tend to be more reactive, because they have fewer valence electrons, which are easier to remove or share with other atoms to form bonds.
Conclusion
So, there you have it, a brief overview of the atom, its structure, atomic number, and atomic mass. The atom is the basic building block of all matter, and understanding its properties and behavior is crucial in many fields, from medicine to energy production. From the number of protons in the nucleus to the properties of isotopes, the atom has many fascinating and important features. We hope you enjoyed learning more about the atom and its components, and that you continue to explore the exciting world of chemistry!
Source image : www.sliderbase.com

Source image : periodictable.me

Source image : awo.aws.org

.PNG)








